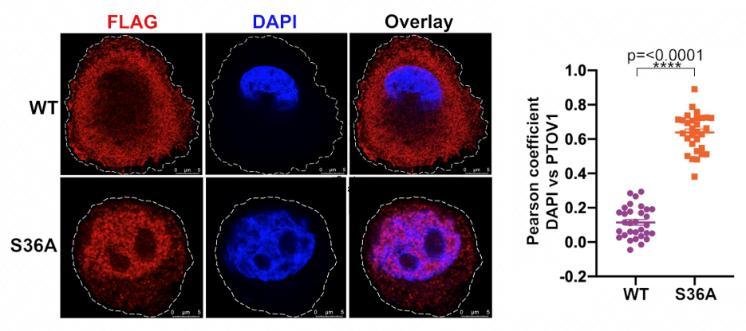
Cells tightly regulate signaling and gene expression patterns to maintain health and viability. When those patterns are disrupted, disease processes such as cancer can develop. The oncoprotein PTOV1 promotes cancer traits such as cell proliferation, motility, and tumor invasiveness through functioning as both a translation factor and transcription factor to regulate gene expression patterns. However, little was known about the signaling and mechanisms regulating PTOV1 function. The authors here present the first known mechanisms regulating the function of PTOV1 within cells. They found that PTOV1 interacts with the 14-3-3 family of phosphobinding proteins, and that this interaction is facilitated by the phosphorylation of PTOV1 by the understudied kinase, SGK2, at serines 36 and 53. The 14-3-3-PTOV1 interaction promotes both retention of PTOV1 in the cytoplasm and protection of PTOV1 from proteasomal degradation mediated by the E3 ligase HUWE1. This retention and stabilization facilitates PTOV1-mediated expression of the oncogenic transcription factor cJun. Altogether, this study elucidates a potentially druggable regulatory mechanism controlling the activity of the oncoprotein PTOV1. Ongoing studies seek to situate this SGK2-PTOV1 within cellular signaling contexts and to further explore the implications of these mechanisms on PTOV1-driven cancer phenotypes.